In surgical manufacturing, quality isn’t optional — it’s life-critical. Every implant, instrument, or device that reaches the operating table must meet the highest levels of precision, safety, and performance. This is where Quality Assurance (QA) steps in as the backbone of modern medical device manufacturing.
In this article, we’ll explore the importance of QA in surgical manufacturing, the global standards governing the industry, and the best practices leading companies like Teton Surgical follow to ensure exceptional product quality and patient safety.
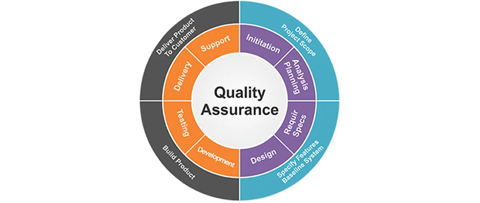
What Is Quality Assurance in Surgical Manufacturing?
Quality Assurance refers to the systematic processes that ensure surgical devices are designed, produced, tested, and delivered according to the highest safety and regulatory standards. It goes beyond simply inspecting finished products — it’s about building quality into every stage of the manufacturing process.
QA in surgical manufacturing covers:
Material selection and traceability
Process validation and control
Risk management
Sterilization assurance
Final inspection and documentation
Regulatory compliance
In a field where millimeters matter, QA ensures that every product performs flawlessly under the most demanding clinical conditions.
Why Quality Assurance Is Non-Negotiable in Medical Device Manufacturing
Surgical implants and tools are used inside the human body. Failure isn’t just expensive — it can be life-threatening.
Here’s why quality assurance is vital:
✅ Ensures Patient Safety
A single defective implant or tool can lead to infection, revision surgery, or even death. QA systems minimize this risk through rigorous testing and validation.
✅ Complies with Regulatory Requirements
Organizations like the FDA (USA), ISO (International), and CE (Europe) require medical manufacturers to maintain detailed quality management systems (QMS).
✅ Boosts Product Reliability and Brand Trust
Hospitals, surgeons, and OEMs trust brands that consistently deliver defect-free, high-performance products. QA builds and maintains that trust.
✅ Reduces Recalls and Legal Exposure
Implementing a proactive QA strategy can prevent costly recalls, production downtime, and legal liabilities.
Key Quality Standards in Surgical Manufacturing
Medical device manufacturing is governed by some of the strictest global standards. Leading companies must align with:
1. ISO 13485:2016 – Medical Device Quality Management System
This is the gold standard for quality systems in surgical manufacturing. It requires:
Full traceability of components
Documented risk assessments
Corrective and preventive actions (CAPA)
Regular audits and quality reviews
2. FDA 21 CFR Part 820 – Quality System Regulation (QSR)
Applicable in the United States, this regulation outlines requirements for design controls, production processes, and record keeping.
3. ISO 14971 – Risk Management for Medical Devices
This standard ensures that every potential risk associated with a surgical device is identified, analyzed, and mitigated before the product reaches the market.
4. Cleanroom & Sterilization Standards
Standards like ISO 14644 for cleanroom environments and ISO 11135 / ISO 11137 for sterilization help guarantee contamination-free manufacturing.
Best Practices for Quality Assurance in Surgical Manufacturing
At Teton Surgical, we implement a comprehensive, proactive approach to quality that spans the entire product lifecycle. Here’s a look at some of the best QA practices in the industry:
1. Incoming Material Inspection
All raw materials are rigorously tested and documented for compliance with mechanical, chemical, and biocompatibility standards.
2. In-Process Quality Checks
Precision inspection tools like coordinate measuring machines (CMMs), optical comparators, and laser micrometers ensure each part meets design specs during production — not just after.
3. Process Validation
All manufacturing processes (CNC machining, welding, surface treatment, etc.) are validated for repeatability and reliability under real-world conditions.
4. End-of-Line Inspection
Before packaging, every surgical instrument or implant is visually and mechanically inspected, ensuring it meets all safety and dimensional requirements.
5. Sterility Assurance
Products undergo validated sterilization methods (ETO, Gamma, Steam) and are packaged in ISO 11607-compliant packaging.
6. Documentation and Traceability
Every product is assigned a unique identifier to trace it back to the raw material batch, machine process, and inspection data — a critical factor in compliance and recalls.
How QA Supports OEM Clients and Innovation
For OEM partners, quality assurance offers more than peace of mind — it supports faster innovation, faster approvals, and market credibility.
At Teton Surgical, we help our partners:
Launch new products with confidence
Navigate complex regulatory pathways
Maintain consistency across global markets
Lower production risks and improve ROI
We work with clients from prototyping through full-scale production, offering both white-label solutions and customized QA protocols tailored to your project needs.
Quality Assurance is the foundation of trust in surgical manufacturing. With lives on the line, there’s no room for shortcuts. Whether you’re developing orthopedic implants or surgical tools, investing in robust QA ensures compliance, consistency, and confidence.





Add comment